|
|||||||||||
|
|||||||||||
| Tin
is a metal in group IV A of the periodic table with atomic number 50, an
atomic weight of 118.69, and a density of 7.7 Mg/m3.
It has a melting temperature of 231.9 C.
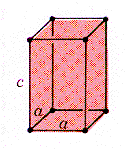
The electronic configuration of tin is (Kr)(4d)10(5s)2(5p)2, and it has an atomic radius of 0.162 nm. At room temperature b-tin (white tin) has a tetragonal crystal structure (shown) with a basis of one tin atom per lattice point. Below 13.5 C, a- tin (gray tin) is the stable form and has the diamond cubic structure with a lattice parameter of 0.495 nm. Tin has a Young's modulus of 44 GPa, a yield stress of 7 MPa, an UTS of 14 MPa, and fracture strain of 0.7. |
|||||||||||
 |
|||||||||||
| From:
Callister,
"Materials Science and Engineering," Wiley (1994) |
|||||||||||