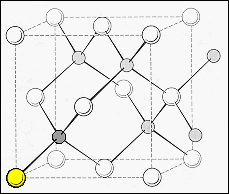
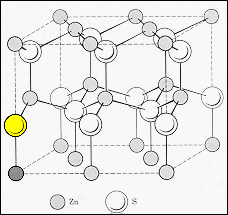
| Zinc
sulphide exists with two different crystal structures, the cubic zinc blend
(top diagram) and the hexagonal Wurtzite (lower diagram).
In
the zinc blend structure the sulphur ions form an fcc structure and the
zinc ions occupy half of the tetrahedral sites in this structure to attain
charge neutrality. The crystal has a lattice parameter of 0.541 nm. The
crystal structure is also known as diamond cubic and may be thought of
as two interpenetrating fcc lattices, one for sulphur the other for zinc,
with their origins displaced by one quarter of a body diagonal.
The
hexagonal wurtzite structure has the sulphur ions in an hcp array and one-half
of the tetrahedral sites in this structure are occupied by zinc ions. The
crystal has a basal lattice parameter, a = 0.233 nm and c/a = 1.63. |