Princeton University
Department of Mechanical and Aerospace Engineering
MAE 324: The Structure and Properties of Engineering Materials
Sample Mid-term
Examination 6
March 2002
One 8.5 x 11” sheet of notes may be used in conjunction with this examination, which is otherwise closed book.
All questions are of equal weight.
Question Grades
1 --------------------------------
2 -------------------------------
3 -------------------------------
Bonus -------------------------
Total --------------------------
Examination Percent -------------------- NormalizedCourse Percent --------------------
Please Copy and Sign:
I pledge that I have not violated the Honor Code during this examination.
Signature: ___________________________________
Name (Please Print) ___________________________
1) An ionic crystal has a cubic Bravais lattice and a basis of two ions. The diagrams below show the ionic configurations of the (100), (110), and (111) planes for this structure. The large circles represent negative ions and the smaller circles positive ions. Each ion has six nearest neighbors of the other kind.
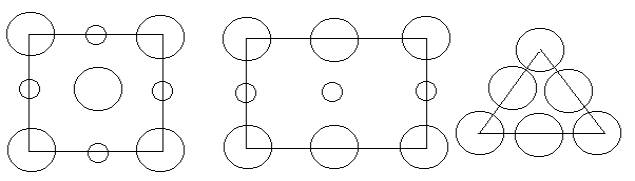
(100) (110) (111)
a) Which of the cubic unit cells describes the Bravais lattice of this crystal?
b) Make a sketch of the Unit cell of the crystal structure and clearly identify one Basis in this cell.
c) How many lattice points does this cell contain?
d) How many ions of each type are contained within the unit cell?
e) Specify the close packed planes of this crystal structure by the correct Miller index, and show one such plane in a dedicated diagram of the unit cell.
f) On this same diagram, show one of the close packed directions of this crystal structure? Specify by the correct Miller index for the selected direction.
2) Aluminum is a face centered cubic metal with extensive technological applications. The material is normally used in a polycrystalline condition.
a) Draw a nominal-stress/nominal-strain curve for a polycrystalline aluminum sample tested in uniaxial tension at a low constant strain-rate. Label the axes and give approximate values (SI units) for the offset yield stress and strain, the ultimate tensile stress and strain, and the fracture stress and strain.
b) On the same diagram, show the true-stress/true-strain response of the material. Compute the values of true-stress and true-strain for the yield stress, ultimate tensile stress, and fracture stress.
c) What change in the behavior of the deforming material occurs at the ultimate tensile stress (UTS)? Make a sketch of a cylindrical sample that has been taken to a strain state just past this value and compare it to the sample prior to reaching the UTS to illustrate this behavior change.
d) If instead of being deformed at constant strain rate, the sample is subjected to a steadily increasing applied load. Draw a nominal-stress/nominal-strain curve for this test condition and explain how and why it differs from the constant strain rate curve.
3) Point defects (Vacancies,
Self-Interstitials, etc..) are thermodynamically favored in pure materials, and
are important in bulk transport processes such as diffusion.
a) Make a sketch of the Gibbs function (free energy) of a metal crystal as a function of the lattice vacancy concentration under constant temperature and pressure conditions. Show on this diagram the contributions due to the enthalpy of vacancy formation and the configurational entropy. Identify on the diagram the expected equilibrium number of vacancies, n0, under these conditions. How will this number change if the temperature is increased keeping the pressure constant? What will be the effect of increasing the pressure at constant temperature?
b) At one atmosphere, the enthalpy associated with the formation of a single vacancy in the fcc metal copper is 0.98 eV/atom. Compute the atomic concentration of vacancies in copper at 1000 C. Boltzmann's constant, k = 8.6 x 10-5 eV/K. State any assumptions made.
c) Self-diffusion in copper takes place via a vacancy mechanism. For this metal,
D0= 7.8 x 10-5 m2 sec-1 and the energy of the diffusion process is Q= 2.18 eV/atom. What is the value of the self-diffusion coefficient of copper at 1000 C?
d) Approximately how far does a copper atom diffuse in 104 sec at this temperature?
Bonus Question (50 points). Identify and briefly
discuss the content of the following photographs and diagrams.
1) 
2)
3)
4)
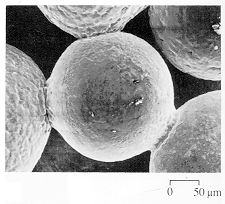
5)