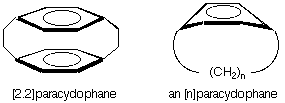
This molecule is a "carboranophane," a carborane analog of a cyclophane. Cyclophanes have a long and varied history. Years ago Don Cram first made [2.2]paracyclophane, a molecule in which two benzene rings were held face-to-face by bridging methylene groups. Later, benzene rings bridged by a single strand of methylene groups were made, and for a while we held the world's record for the shortest chain (n = 6 and 7). Our carbene route to [n]cyclophanes has now been eclipsed by beautiful work by the groups of F. Bickelhaupt in Holland and Yoshito Tobe in Japan who were able to make molecules with even smaller bridges. Evidence for [4]paracyclophane now exists. Much of the interest in [n]cyclophanes stems from the question of how the benzene ring, which surely prefers planarity, will cope with the bending strain induced by the bridge.
The carboranes are three-dimensional analogs of benzene. Thus the question arises of how the rigid cage will react to bridging by a short chain of carbon. The molecule shown represents out first efforts to expand our traditional cyclophane work to these boron-carbon cages. Our synthesis of the carboranophane is shown below. Notice that Lisa Barnett-Thamattoor's synthesis provides a bonus in the isolation of a (remote) analog of Cram's [2.2]paracyclophane as an additional product.
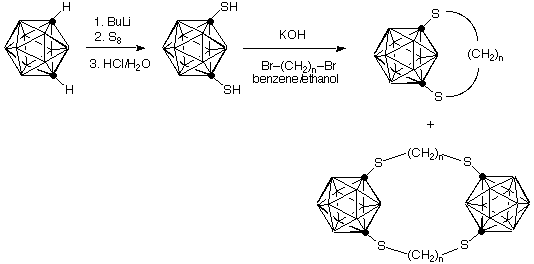
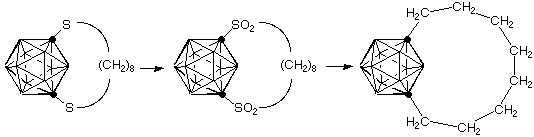
Want more details? See:
Carboranophanes. Barnett-Thamattoor, L.; Wu, J. J.; Ho, D. M.; Jones, M. Jr. Tetrahedron Lett. 1996, 37, 7221.