Research: Aluminum Solubility in the Presence of Citric Acid
Daniel M. Dabbs
Aluminum contamination of soil and ground water and the possible connection to a wide range of human ailments has led to increased interest in the speciation of aluminum within natural waters,[1] soil,[2] and blood serum.[3] As a result, interest centers on the physiological pH range of 6-9,[4,5] and information on the solubility of aluminum in high pH solutions (pH ≥ 10), conditions of high hydrolysis, is limited primarily to the prevalent aluminum-containing species observed in thermodynamically stable solutions.[6] Despite this apparent lack of background information, the speciation of aluminum under high pH conditions is important in the remediation of water-based solutions of hazardous waste materials, especially those found in tanks of radioactive wastes.[7]
Soluble aluminum oxyhydroxides and insoluble aluminum-containing particles complex with citric acid under conditions of high hydrolysis—hydroxide ion to aluminum ratios in excess of 2.46 mol/mol.[8] The organic can act to protect existing particle surfaces from dissolution or to maintain cation solubility. We have titrated solutions of aluminum chloride with NaOH (aq), adding citric acid in amounts ranging from zero to slightly less than citrate/Al = 1 mol/mol.
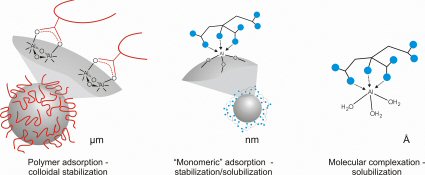
Mechanisms of molecular complexation and colloidal stabilization. Interactions between the complexing agent and the target atom scales from the microscale (surfaces) to the atomic. “Stability”, the continued existence of particle or molecule, can be imparted either thermodynamically or kinetically.
Changes in the size of suspension particles were measured using quasi-elastic light scattering. Solutions of aluminum chloride with no citric acid added formed large particles that quickly shrank until sufficient hydroxide was added and particles remained in suspension. Large aluminum-containing polycations were detected by 27Al solution nmr under conditions of high hydrolysis, and these may have provided seeds for particle formation.
The addition of citric acid changed the dynamics of both solutions and suspensions. In solutions of very high hydrolysis (OH/Al = 3.29), particles did not form when the mole/mole ratio of citrate to aluminum equaled 0.8. Under the same hydrolysis condition but at citrate/Al ratios less than 0.8, particles formed but nucleation and growth were markedly slowed. Under conditions of high hydrolysis (OH/Al = 2.46), particles formed in the presence of citric acid when base was added but the rate of formation was slowed. Polycations were not detected in solutions containing citric acid, suggesting that important seeds or building blocks in particle formation and growth were eliminated by the interactions of citric acid with aluminum centers.
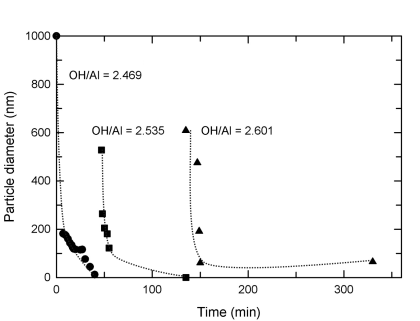
When adding NaOH (aq) to solutions of AlCl3, particles are unstable for OH/Al less than 2.6. Particles quickly form and reach large size, then shrink (or deaggregate) and disappear. At OH/Al above 2.6, particles shrink to a stable size and the resulting suspension remains stable at room temperature.
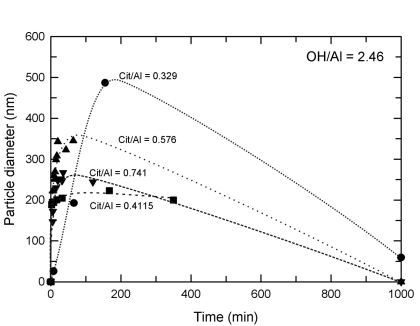
Particle growth remains rapid but subsequent diminution is slowed in the presence of citric acid under conditions of high hydrolysis. At higher OH/Al contents, particle growth can be stopped using sufficient citric acid (~0.8 citric acid to aluminum by mole).
References
1. B.C. Faust, W.B. Labiosa, K.H. Dai, J.S. MacFall, B.A. Browne, A.A. Ribeiro, D.D. Richter Geochim. Cosmochim. Acta 1995 59 2651-61.
2. D.L. Jones Plant and Soil 1998 205 25–44; J.F. Ma, P.R. Ryan, E. Delhaize Trends Plant Sci. 2001 6 273-8.
3. A. Lakatos, F. Evanics, G. Dombi, R. Bertani, T. Kiss Eur. J. Inorg. Chem. 2001 12 3079-86.
4. L.-O. Öhman Chem. Geol. 1998 151 41–50.
5. R.B. Martin Acc. Chem. Res. 1994 27 204-210; L.-O. Öhman, R.B. Martin Clin. Chem. 1994 40 598-601.
6. A. Lakatos, I. Bányai, P. Decock, T. Kiss Eur. J. Inorg. Chem. 2001 2 461-9.
7. Tanks Focus Area, 1997 Annual Report (National Technical Information Service, U.S. Department of Commerce, Springfield, VA 1998).
8. D.M. Dabbs, U. Ramachandran, S. Lu, J. Liu, I.A. Aksay research in progress (2004).
For more information, please contact Daniel Dabbs. See the Ceramic Materials Laboratory web page for more information about our group and its research.
![]()
![]()
![]() © 2004 Princeton University, Ceramic Materials Laboratory.All Rights Reserved.
© 2004 Princeton University, Ceramic Materials Laboratory.All Rights Reserved.