Research: Self-Healing Materials Using Electrohydrodynamics
Mike A. Slowik
Advisors: Dudley A. Saville and Ilhan A. Aksay
When stress is applied to a system in man-made materials failure of the material occurs shortly after the initial defect forms. There are several applications where the ability to prevent or postpone failure after the initial defect would be extremely useful. Currently there are only a very limited amount of materials which exhibit these self-healing characteristics. Two examples are SiC [1] and MoSi2 heating elements, which form a protective silica coating on their surface after being scratched. However, this process occurs only at extremely high temperatures and is thus not suitable in many applications.
To increase the life span of materials following damage new methods involving electrohydrodynamics are being explored. This technology is being tested initially with a system that contains two cylinders, one inside the other, with an electrical current applied to the system as shown in the following schematic. Between the two cylinders is a colloidal dispersion of polystyrene particles, which are used to repair defects that occur in the inner cylinder. When a defect occurs the current density at the damaged site is increased, causing colloidal particles to coagulate around the defect [2].
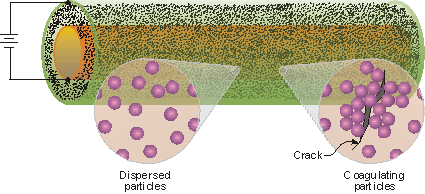
A schematic for a self-healing system that uses the electrohydrodynamic coagulation of particles to close a defect in a cylinder wall.
The defect is not completely repaired because the voids between the colloids prevent the formation of a completely dense surface. To fill in these interstitial spaces various salts are also dissolved in the colloidal dispersion (e.g., NiSO4) and these electrochemically deposit within the voidal spaces ultimately filling the void [3]. This process produces a coating of a ceramic/metal composite at the site of the initial defect.
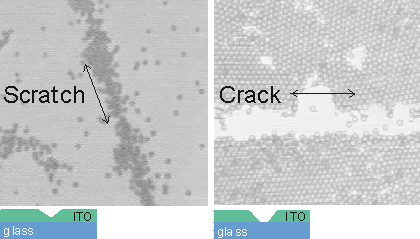
Optical microscope images of colloidal particles (2Ám polystyrene) forming on an ITO coating on a glass substrate. (Left) A scratch that does not penetrate to the substrate encourages the preferential coagulation of particles at the scratch. (RIght) However, a penetrating crack prevents particle coagulation even under conditions favoring the formation of 2-dimensional crystalline layers of particles. (Micrographs courtesy of William Ristenpart)
References
![]() 1.
N. Yao, A. Y. Ku, N. Nakagawa, T. Lee, D. A. Saville, and I. A. Aksay,
"Disorder-Order Transition in Mesoscopic Silica Thin Films," Chem.
Mater. 12 [6] 1536-548 (2000).
1.
N. Yao, A. Y. Ku, N. Nakagawa, T. Lee, D. A. Saville, and I. A. Aksay,
"Disorder-Order Transition in Mesoscopic Silica Thin Films," Chem.
Mater. 12 [6] 1536-548 (2000).
![]() 2.
M. Trau, D. A. Saville, and I. A. Aksay, "Assembly of Colloidal Crystals
at Electrode Interfaces," Langmuir 13 [24] 6375-81
(1997).
2.
M. Trau, D. A. Saville, and I. A. Aksay, "Assembly of Colloidal Crystals
at Electrode Interfaces," Langmuir 13 [24] 6375-81
(1997).
3. P. Sakar, X. Huang, O. Prakash, and P. Nicholson, "Electrophoretic Deposition to Synthesize Advanced Ceramic/Ceramic Laminar Composites," in Advances in Ceramic-Matrix Composites N. P. Bansal, ed. (American Ceramic Society:Westerville, Ohio, 1993) p. 39.
For more information, please contact Mike Slowik. See the Ceramic Materials Laboratory web page for more information about our group and its research.
![]()
![]()
![]() © 2004 Princeton University, Ceramic Materials Laboratory.All Rights Reserved.
© 2004 Princeton University, Ceramic Materials Laboratory.All Rights Reserved.