Research: Silicon Nitride Ceramics
David L. Milius and Hsien-Liang Ker*
now with AVX Corporation, Myrtle Beach, South Carolina
This research investigates the surface chemistry of silicon nitride in order to determine the characteristics of ionic non-ionic polymer pairs that could be adsorbed on silicon nitride in order to induce plasticity in the consolidated state. Following the selection of the ionic non-ionic polymers the experimental procedure was to optimize the plasticity using a commonly available silicon nitride. Plastic silicon nitride can be used to form ceramic parts for mass production, represented by the silicon nitride bearing shown in the accompanying figure.

Green-body bearing (left) and sintered bearing (right) after sintering and polishing. The 'belly band' on the green body is a result of the forming technique and must be removed prior to sintering.
Both single-chained and double-chained cationic surfactants are used to convert the surface of silicon nitride to be hydrophobic. Non-ionic octylphenol polyether alcohol (OPEx) surfactants having different hydrophilic chain lengths are then adsorbed on the hydrophobic surface. The measured forces were well related to the rheology and consolidation behavior of the suspensions. Short-chained OPE1 and OPE3, were found to adsorb on the hydrophobic silicon nitride surface despite their insolubility in water. Attraction between ethylene oxide chains of OPE surfactants, whose cloud points are below room temperature, are shown to have a significant contribution to the overall inter-particle force. This can possibly lead to a weak attraction between particles which is considered to be essential for plasticity in the consolidated green bodies.
The adsorption of surfactant aggregates on silicon nitride surface from a cationic/nonionic surfactant mixture and its effect on the inter-particle forces and colloidal properties are the focus of this research. Cationic surfactants replace the role of the anionic surfactants because of the lower iso-electric point of silicon nitride surface (pH 6-7) compared with that of alumina (pH 8-10). The results of force measurement indicate the attraction between the ethylene oxide chains of some nonionic surfactants, whose cloud points are below room temperature, can also be a significant portion of the total force. The correlation between the measured inter-particle forces and the observed colloidal properties show us insight into these systems and provide us the capability to design the colloidal systems whose properties will meet the specified requirement.
A closely-packed nonionic surfactant aggregate layer can form on top of hydrophobic silicon nitride surface pre-adsorbed with a cationic surfactant aggregate layer, even when the nonionic surfactant is not soluble in water. Only at high adsorption densities can these surfactant aggregate layers possess rupture force high enough to prevent particles from coming into hydrophobic contact. Under this circumstance, the silicon nitride particle suspensions have low viscosity and can be consolidated to high-density green bodies. Attraction between ethylene oxides chains of OPE surfactants, whose cloud points are below room temperature, is shown to have a significant contribution to the overall inter-particle force. It can possibly lead to weak attraction among particles which is considered to be essential for plasticity in the consolidated green bodies. High solid loadings are possible as shown in the following figure.
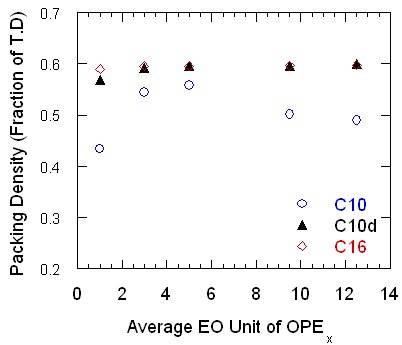
High packing densities are a result of appropriate lubrication of the particle surface
References
1. H.-L. Ker, Ph.D. Dissertation, Princeton University (2001).
2. I. W. Osborne-Lee and R. S. Schechter, "Nonideal Mixed Micelles: Thermodynamic Models and Experimental Comparison," pp. 30-43 in ACS Symp. Series 311, Phenomena in Mixed Surfactant Systems, edited by J. F. Scamehorn (Am. Chem. Soc., Washington, DC, 1986).
2. M. J. Rosen, "Molecular Interaction and Synergism in Binary Mixtures of Surfactants," pp. 144-62 in ACS Symp. Series 311, Phenomena in Mixed Surfactant Systems, edited by J. F. Scamehorn (Am. Chem. Soc., Washington, DC, 1986).
3. M. Abe and K. Ogino, "Solution Properties of Anionic-Nonionic Mixed Surfactant Systems," pp. 1-22 in Mixed Surfactant System, edited by K. Ogino and M. Abe (Marcel Dekker, New York, NY, 1993).
4. K. Motomura, H. Matsukiyo, and M. Aratono, "Thermodynamic Study of the Surface Adsorption and Micelles Formation of Mixed Surfactants," pp. 163-71 in ACS Symp. Series 311, Phenomena in Mixed Surfactant Systems, edited by J. F. Scamehorn (Am. Chem. Soc., Washington, DC, 1986).
5. M. J. Rosen, "Synergism in Binary Mixtures of Surfactants at Various Interfaces," pp. 316-26 in ACS Symp. Series 501, Mixed Surfactant Systems, edited by P. M. Holland and D. Rubingh (Am. Chem. Soc., Washington, DC, 1986).
6. P. M. Holland, "Nonideality at Interfaces in Mixed Surfactant Systems," pp. 327-41 in ACS Symp. Series 501, Mixed Surfactant Systems, edited by P. M. Holland and D. Rubingh (Am. Chem. Soc., Washington, DC, 1986).
7. J. H. Harwell and J. F. Scamehorn, "Adsorption from Mixed Surfactant Systems," pp. 263-81 in Mixed Surfactant System, edited by K. Ogino and M. Abe, Marcel Dekker (New York, NY, 1993).
8. K. Ogino and M. Abe (Eds), Mixed Surfactant System (Marcel Dekker, New York, NY, 1993).
9. P. M. Holland and D. Rubingh (Eds), ACS Symp. Series 501, Mixed Surfactant Systems (Am. Chem. Soc., Washington, DC, 1986).
10. J. F. Scamehorn (Ed), ACS Symp. Series 311, Phenomena in Mixed Surfactant Systems (Am. Chem. Soc., Washington, DC, 1986).
11. J. F. Scamehorn, R. S. Schechter, and W. H. Wade, "Adsorption of Surfactants on Mineral Oxide Surfaces from Aqueous Solutions; III. Binary Mixtures of Anionic and Nonionic Surfactants," J. Colloid Interface Sci. 85 [2] 494-501 (1982).
12. K. Esumi, Y. Sakamoto, and K. Meguro, "Mixed bilayers of Anionic and Nonionic Surfactants on Alumina," J. Colloid Interface Sci. 134 [1] 283-88 (1990).
13. E. Fu, P. Somasundaran, and Q. Xu, "Thermodynamic Study of Adsorption of Anioic-Nonionic Surfactant Mixture at the Alumina-Water Interface," pp. 366-76 in Mixed Surfactant Systems, ACS Symp. Series 501, edited by P. M. Holland and D. N. Rubingh (Amer. Chem. Soc., Washington, D.C., 1992).
For more information, please contact David Milius. Additional information concerning the CML can be found on our website.
![]()
![]()
![]() © 2001 Princeton University, Ceramic Materials Laboratory.All Rights Reserved.
© 2001 Princeton University, Ceramic Materials Laboratory.All Rights Reserved.