Research: Ceramic/polymer Composite Materials through Stereolithography
Jim H. Lee
Advisors: Robert K. Prud'homme and Ilhan A. Aksay
The aim of the biomaterials effort is to produce bone graft substitutes that mimic the properties of bone. As pure ceramic proves to be too "brittle" for bone, and polymer, too weak, it has been proposed that a composite of the two materials would provide a better match with natural bone.
Natural bone.
Our approach has taken a two-pronged attack. The initial goal was to form fully ceramic compacts by using low concentration water-soluble polymers as intermediate binders in the process of stereolithography. Stereolithography (SL) (see figure below) enables the formation of a 3-dimensional object through the successive photocuring of polymer layers [1,2]. The challenge, then, is to understand the effect of ceramic inclusions on stereolithography in aqueous media.
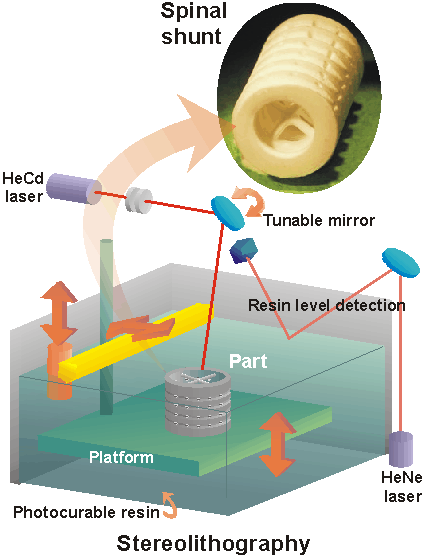
Schematic of stereolithography apparatus with an example of a ceramic spinal shunt built using ceramic stereolithography (CSL).
The introduction of ceramic inclusions into the photocurable suspension causes multiple scattering effects that result in a diffusive beam profile as the mean transport path of photons, ltr, into the solution decreases. With the assumption that interference effects in the medium (modeled here as semi-infinite) can be neglected, a complete description of photon transport can be made using a Percus-Yevick structure factor. Comparison between theory and experimental data are shown [3,4] below for increasing volume fraction ceramic, f.
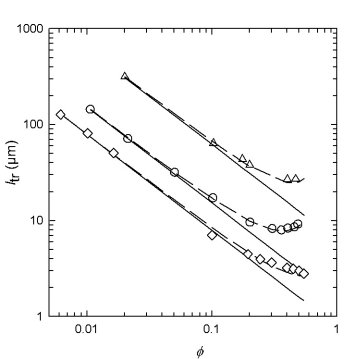
Transport mean free path versus volume fraction, f, of scattering particles on log-log scale. Solid curves are computed from Percus-Yevick theory. Symbols represent transport lengths obtained from experiment: diamonds represent 0.51 Ám alumina, triangles represent 0.46 Ám silica, and circles represent 0.32 Ám alumina, and. The curve for 0.51 Ám alumina has been shifted up by log(3) for clarity.
Once the effect of filler on the beam profile was understood, we then focused our efforts on developing the proper composite systems with regards to biocompatibility and mechanical performance. Design of the composite first begins with theoretical considerations of mechanical performance[5-7]. With these guidelines in hand, recent work by Jim Lee has focused on the dispersion of ceramic material in a non-aqueous system. Polymer is added to the system to provide the desired polymer/ceramic volume ratio.
Crucial to this stage is the development of high concentration biocompatible resins in non-aqueous media. The problem quickly becomes one of understanding and optimizing the curing behavior of the resin systems in solution. We therefore developed a new quantitative model for the curing properties in non-aqueous medium that can be used to predict the depth of cure, the subject of a recent article submission[8-11]. Interestingly, there exists an optimal photoinitiator concentration for which the cure depth is maximized, as shown below.
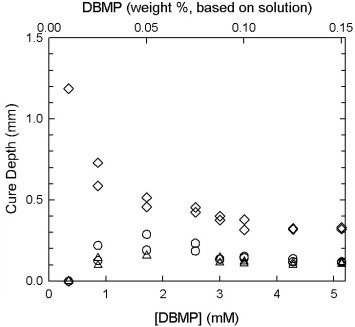
Gel thickness versus photoinitiator concentration. The three data curves correspond to the three different laser energy dosages (diamonds for 22.255 J/cm2, circles for 1.702 J/cm2, and triangles for 0.931 J/cm2). Photoinitiator concentration is given in millimoles/liter on the bottom abscissa, and as weight percent based on total solution on the top abscissa. Note the existence of an optimal photoinitiator concentration that maximizes cure depth.
Coupling the aforementioned phenomena, we are now able to fabricate prototype composites, as shown in the figure below.
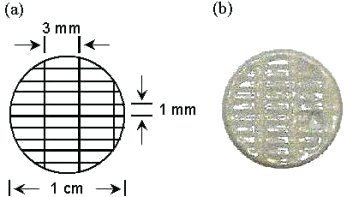
(a) CAD schematic of UV-cured disc. Black lines represent cross-hatched laser rastering pattern (laser beam diameter of 250 Ám and wavelength of 325 nm); (b) Bis-GMA/alumina (10/90 by volume) thin film composite formed in the SLA. Note that the designed architecture of the CAD file has been reproduced successfully.
New efforts have sprung from these efforts. One is the consideration of pore size effects in facilitating cell growth [12]. While the development of a biocompatible composite has been demonstrated, it is desired to fabricate implant materials that are not only osteoconductive, but osteoinductive.
References
1. P.F. Jacobs, Rapid Prototyping & Manufacturing (Soc. of Manufacturing Engineers, Dearborn, MI 1992).
2. P.F. Jacobs, Stereolithography and other RP&M Technologies (Soc. of Manufacturing Engineers, Dearborn, MI 1996).
![]() 3. R. Garg,
R.K. Prud'homme, I.A. Aksay, F. Liu, and R. Alfano, J.
Opt. Soc. Am. 15 (1998).
3. R. Garg,
R.K. Prud'homme, I.A. Aksay, F. Liu, and R. Alfano, J.
Opt. Soc. Am. 15 (1998).
![]() 4. R.
Garg, R.K. Prud'homme, I.A. Aksay, F. Liu, and R. Alfano, J.
Mater. Res. 13 (1998).
4. R.
Garg, R.K. Prud'homme, I.A. Aksay, F. Liu, and R. Alfano, J.
Mater. Res. 13 (1998).
5. Z. Hashin and S. Shtrikman. J. Mech. Phys. Solids 11 127-140 (1963).
6. J. Halpin, J. Composite Mater. 3 732 (1969).
7. L. Nielsen, J. Appl. Phys. 41 4626-7 (1970).
8. J.H. Lee, R.K. Prud'Homme, and I.A. Aksay, Proceedings of the Materials Research Society: Solid Freeform Fabrication and Additive Processing III 625 (MRS, Warrendale, PA, 2000).
9. J.H. Lee, R.K. Prud'homme, and I.A. Aksay, J. Mater. Res. (submitted August 2000).
10. P. Bernhard, M. Hofmann, A. Schulthess, and B. Steinmann, Chimia 48 427-30 (1994).
11. K.S. Anseth, S.M. Newman, and C.N. Bowman, Adv. Polym. Sci. 122 177-217 (1995).
12. L.L. Hench, "Bioceramics," J. Am. Ceram. Soc. 81 [7] 1705-28 (1998).
For more information on this research topic, please contact Jim H. Lee or see the Ceramic Materials Laboratory web page for more information and recent publications.
![]()
![]()
![]() © 2001 Princeton University, Ceramic Materials Laboratory.All Rights Reserved.
© 2001 Princeton University, Ceramic Materials Laboratory.All Rights Reserved.