Solids: Thermodynamics and Bonding |
|
||||||||||||||
|
|
|
|||||||||||||
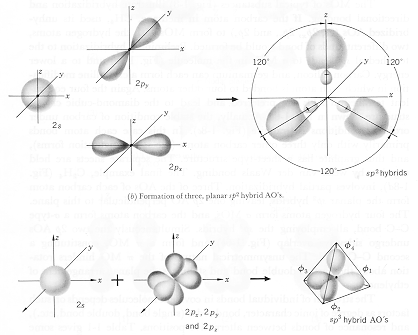
· Electron Distributions associated with more than one atomic
state may combine to form "Hybridized" molecular bonds. |
|||||||||||||||
 |
|||||||||||||||
From: Guy, "Introduction to Materials Science," McGraw Hill (1972) |
|||||||||||||||