Research: Electrically Guided Assembly of Planar Superlattices
William D. Ristenpart
Advisors: Dudley A. Saville and Ilhan A. Aksay
Monodisperse colloidal particles exhibit a wide range of behavior in electric fields near electrodes.[1] Application of DC or low frequency (f < 1 kHz) AC fields causes particles to move laterally across an electrode, yielding planar close-packed clusters.[1-3] Electrohydrodynamic (EHD) fluid flow has been invoked to explain this behavior.[4–6] In high frequency (f > 1 kHz), high amplitude fields, dipole-dipole interactions produce widely spaced arrays.[7,8] At intermediate frequencies, the attractive and repulsive forces balance, as evidenced by the formation of clusters with average interparticle separations of several radii.[9,10] The ability to tune particle interactions simply by adjusting the frequency has implications for both device fabrication[11] and fundamental studies. We have described the behavior of suspensions with two sorts of particles with similar radii (a "binary" suspension) subjected to an AC field.[1] We have demonstrated that the AC field produces new morphologies—"strings" of alternating particle types and triangular- and square-packed superlattices—over a range of frequencies. This behavior has been explained in terms of EHD flow and induced-dipole interactions.[1]

Optical images of planar structures formed with binary suspensions on an electrode in low frequency electric fields. Dark particles are 2:0-µm diameter polystyrene and light particles are 1:8-µm diameter silica; the field is oriented out of the page. (a) Random arrangement before application of a field. (b) Lateral separations induced with a 10 kHz, 40 V/mm field. (c) Individual silica particles typically surrounded by six polystyrene particles formed in a 3 kHz, 40 V/mm field with a local 8:1 ratio of polystyrene to silica. (d) Structures formed in a 3 kHz, 40 V/mm field with a local 2:1 ratio of polystyrene to silica. Note the region with triangular superlattices coexistent with a region of alternating lines of particles.[1]
The crystallization process is largely independent of the field strength above a threshold of ~5 V/mm (2 ≤ f ≤ 10 kHz). Changing the field strength at constant frequency results in little or no discernible change in the lateral separation between particles, irrespective of whether they are closely aggregated (as at 2 kHz) or widely separated (as at 10 kHz). The absence of field strength dependence suggests that repulsive and attractive interaction forces scale with field strength in the same fashion. Monodisperse suspensions behave in the same way under these conditions indicating that the cause of aggregation is not intrinsically linked to a binary character. Various superlattice structures form, reproducibly, in independent experiments and various cluster morphologies are observed: ringed clusters, striped clusters, disordered clusters, and regions where the two types of particles are completely segregated. Different morphologies are often present on adjacent regions of the electrode, suggesting that factors other than the electric field and particle concentration may play a role.

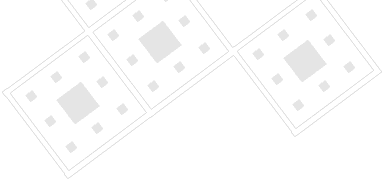
Optical images of planar structures formed with binary suspensions on an electrode in high frequency electric fields. The field strength is 60 V/mm at 100 kHz, oriented out of the page. Particle characteristics are the same as in the first figure. (a) Chains of alternating particle type with a 1:1 ratio of polystyrene to silica (20% total area coverage). (b) Triangular superlattices formed with a 2:1 ratio of polystyrene to silica (80% total area coverage). (c) Square-packed superlattices formed with a 1:1 ratio of polystyrene to silica (80% total area coverage).
It is unlikely that EHD flow alone produces a superstructured arrangement. Although EHD flows are sensitive to particle size—since the particles serve to disrupt the concentration polarization layer near an electrode— they are insensitive to other particle characteristics. As the particles used in this work are of a similar size, the small size difference appears insufficient to produce superstructure. The (repulsive) induced-dipole interaction energy between polystyrene particles, however, is about twice the silica-polystyrene or silica-silica energies and less energetically favored (see below). Given that EHD flow draws particles into close proximity, superlattices can be interpreted as structures that somehow lessen the total interaction energy by lowering the number of strongly repulsive interactions.
Briefly, we have demonstrated that electric fields can be used to modulate the interaction energies of particles with different polarizabilities and have developed a theoretical framework for the interaction forces over a wide range of frequencies.[1] At certain frequencies and relative particle concentrations, a diverse assortment of planar superstructures is obtained. The electric-field approach presented here might also be extended to multicomponent colloidal suspensions. Techniques for guiding the long-range organization of particles, such as those based on physically imposed particle confinement or UV light directed particle motion, could be used in conjunction with this technique to improve long-range order.
References
1. W.D. Ristenpart, I.A. Aksay, D.A. Saville Phys. Rev. Lett. 90 [12] (2003).
2. M. Trau, D.A. Saville, I.A. Aksay Science 272 706 (1996).
3. M. Bohmer Langmuir 12 5747 (1996).
4. M. Trau, D.A. Saville, I.A. Aksay Langmuir 13 6375 (1997).
5. P.J. Sides Langmuir 17 5791 (2001).
6. Y. Solomentsev, M. Bohmer, J.L. Anderson Langmuir 13 6058 (1997).
7. R.E. Kusner et al. Phys. Rev. Lett. 73 3113 (1994).
8. T. Gong and D.W.M. Marr Langmuir 17 2301 (2001).
9. J. Kim et al. Adv. Colloid Interface Sci. 96 131 (2002).
10. F. Nadal et al. Phys. Rev. E 65 061409 (2002).
11. G.M.Whitesides and B. Grzybowski, Science 295 2418 (2002); K. H. Lin et al. Phys. Rev. Lett. 85 1770 (2000); A. van Blaaderen, R. Ruel, P. Wiltzius Nature 385 321 (1997).
For more information on this research topic, please contact Bill Ristenpart. Additional information concerning the CML can be found on our website.
![]()
![]()
![]() © 2004 Princeton University, Ceramic Materials Laboratory.All Rights Reserved.
© 2004 Princeton University, Ceramic Materials Laboratory.All Rights Reserved.