|
|
|
||||||||||||||
The Structure of Solids |
||||||||||||||||
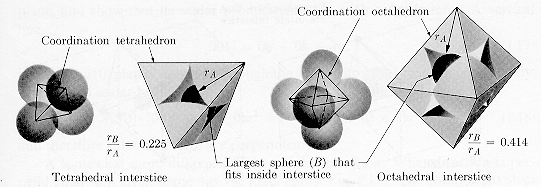
· The octahedral and tetrahedral
interstitial
sites of an fcc
or hcp
structure are shown. There is a part of an atom associated
with each vertex and an interstitial site at the center of each polyhedron. |
||||||||||||||||
 |
||||||||||||||||
From: Guy and Hren, "Elements of Physical Metallurgy," Addison Wesley (1974) |
||||||||||||||||