|
|
|
|||||||||||||||
Phase Diagrams |
|||||||||||||||||
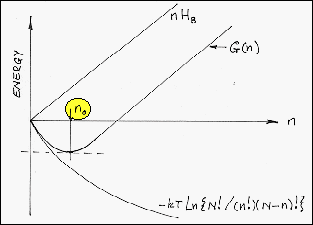
· The Gibbs
function for a substitutional
solid solution of B-atoms in an A-atom matrix depends upon the number
of B-atoms in solution and their enthalpy of solution. This is illustrated
below. |
|||||||||||||||||
· If HB = Enthalpy
of solution of B-atoms in an A-atom matrix, N = number of lattice sites, n =
number of B-atoms, (N - n) = number of A-atoms, both the enthalpy and the entropy
of the alloy depend upon n. |
|||||||||||||||||
 |
|||||||||||||||||
· The B-atom equilibrium concentration,
n0, is then given by: |
|||||||||||||||||