| Solids: Thermodynamics and Bonding |
|
||||||||||||||
|
|
|
|||||||||||||
| ·
Among
the bonding types, Ionic, Metallic, and van der Waals bonds are non-directional
and depend only on the interatomic separation.
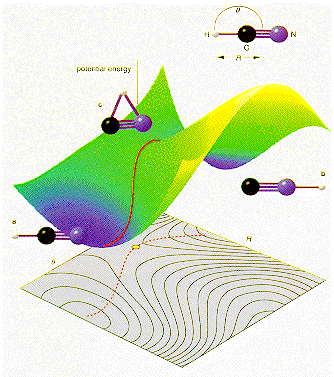
· Covalent and Hydrogen bonds are directional, and an angular distortion increases the energy of the system. · The effect of changing bond angle is illustrated for the HCN molecule. The effect of changing R is also shown on this potential diagram. · In general, more than one bonding type will play a role in the cohesion of most solids. |
|||||||||||||||
 |
|||||||||||||||
| From: Carpenter, "Reaction Dynamics in Organic Chemistry," American Scientist (March 1997) 139 | |||||||||||||||