Solids: Thermodynamics and Bonding |
|
||||||||||||||
|
|
|
|||||||||||||
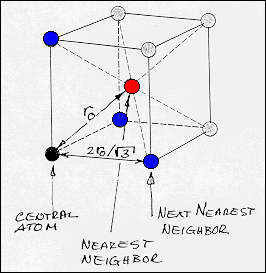
· The unit cell in the diagram shows the relative positions
of the metal ion-cores in metallic lithium. |
|||||||||||||||
 |
|||||||||||||||
· The interaction of the electrons with more than one
ion-core
reduces their potential energy, and the increased volume of space they occupy
decreases their kinetic energy as compared to the bound 2s electrons. |
|||||||||||||||