Solids: Thermodynamics and Bonding |
|
||||||||||||||||
|
|
|
|||||||||||||||
METALLIC
BONDING |
|||||||||||||||||
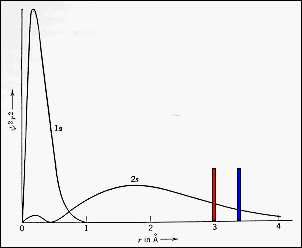
· Lithium may be considered as a prototype metal and has the
electronic configuration: (1s)2(2s)1 . The electron
distributions are shown in the diagram. |
|||||||||||||||||
 |
|||||||||||||||||
From: Sproull, "Modern Physics," Wiley (1956) |
|||||||||||||||||