| |
· The van der Waals potential is a function of r only, and
has the Lennard-Jones form: V(r) = - (A/r6 ) + (B/r12 )
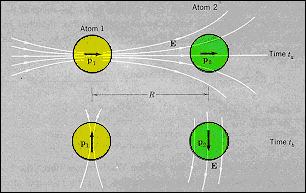
· A may be written in terms of the dipole moments, p, and the
polarizability, α, of the atoms: p2 =αE = 2αp1
/r3, where E is the dipole electric field at
atom 2 due to the instantaneous dipole on atom 1, so that: (A/r6 ) =
(2p1 p2 /r3 ) = (4αp12 /r6 )
· This attractive potential is both short range and weak.
· The repulsive potential is also short range, but increases
rapidly as distance decreases. |
|
|
|