| |
Permanent
Dipoles
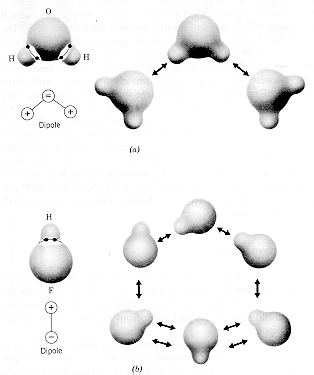
· Two examples of bonding due to permanent
molecular dipoles are shown in the diagram.
· In both cases,
the hydrogen component of the dipole has a positive charge because the electron
distribution is more centered on the oxygen or fluorine components of the
molecules.
· Because specific molecular locations are positive
or negative, the bonding due to the dipolar interaction is directional.
· In ice, this directionality gives rise to many different
crystal structures. |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
From:
Moffatt, Pearsall, and Wulff, "The Structure and Properties of Materials,"
Wiley (1967) |
|
|
|
|
|
|