|
Protection
Methods
·
In
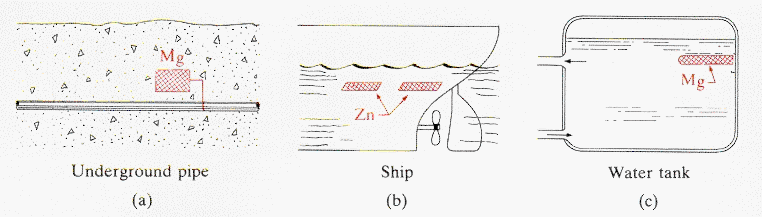
electrochemical corrosion, it is the anode at which the dissolution of
the material occurs. The diagram below shows three situations in which
systems are protected by providing a sacrificial anode that is electrically
connected to the material to be protected.
· In
(a) a steel pipe is protected by use of a magnesium plate connected to
the pipe. The electrode potential of Mg is - 2.38 V and of Fe -0.44 V,
the Mg, therefore, forming the anode of this electrochemical cell. The
same strategy is shown in (b) for protecting a ship hull against corrosion
in sea water, and (c) shows a Mg anode protecting a steel water tank.
·
These
anode structures are replaceable and protect the cathodic component as
long as they are present. |