|
|
|
||||||||||||
| The Structure of Solids | ||||||||||||||
| Covalently
Bonded Ceramics
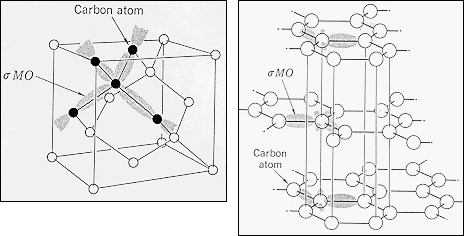
· Diamond
& Graphite are both carbon "Ceramic" materials, Diamond with hybridized
(sp3 ) bonding and Graphite with hybridized
(sp2 ) bonding.
|
||||||||||||||
| ·
Diamond
has an fcc lattice with a basis of two carbon atoms (Diamond Cubic).
· Graphite has a layer structure with covalent bonding in the plane and van der Waals bonds between the planes. |
 |
|||||||||||||
| After: Kingery et al, "Introduction to Ceramics," Wiley (1976) | ||||||||||||||