| Corrosion & Environmental Degradation |
|
|||||||||||||
|
|
|
||||||||||||
| ·
Dynamic
equilibrium between the metal and the electrolyte involves thermally activated
processes.
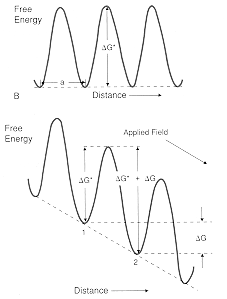
· The metal ions overcome a free energy barrier to enter the electrolyte and become hydrated ions and these must dissociate to permit the metal ion to return to the metal. · At zero applied electric field, the forward and backward rates of ion transfer are the same. Exchange currents of equal magnitude and opposite direction flow between the metal and the electrolyte. Their magnitude depends on the reacting system. Values for several systems are shown: Ag+ +e <-> Ag , ie = 10+4 A/m2 Cu2+ + 2e <-> Cu , ie = 10 A/m2, Fe2+ + 2e <-> Fe, ie = 10 -4 A/m2 · The diagrams show how an applied field will alter the magnitude of the barrier that has to be overcome in the forward and backward directions, favoring one direction of transfer, and causing a net current to flow. |
||||||||||||||
 |
||||||||||||||