| Corrosion & Environmental Degradation |
|
|||||||||||||
|
|
|
||||||||||||
| ·
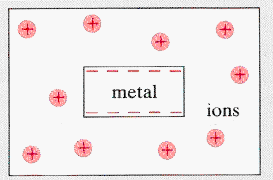
The
steady-state condition between a metal and an electrolyte is shown. In
equilibrium, positive metal ions leave the metal which thus acquires a
net negative charge. Ion exchange is always taking place.
· The system free energy is reduced below that of the isolated metal due to the entropy increase, ΔS, associated with the solution process. This is balanced by the enthalpy increase, ΔH, required to create the ions. In the steady state, these two contributions are equal and opposite, so that: ΔH = TΔS and ΔG is zero for ionic exchange in steady state. · In an aqueous electrolyte, the ions can attract polar water molecules to form a hydrated species. The overall value of ΔH decreases for the reaction. More ions can, therefore, be formed before steady-state is reached . · If the electrolyte is a solution of a salt of the metal, the added entropy contribution from dissolving more metal ions is reduced logarithmically. Fewer ions from the metal will be required to achieve ΔH = TΔS. |
||||||||||||||
 |
||||||||||||||
| From:
Newey and Weaver,
"Materials Principles and Practice," Butterworths (1990) |
||||||||||||||