| Corrosion & Environmental Degradation |
|
||||||||||||||
|
|
|
|||||||||||||
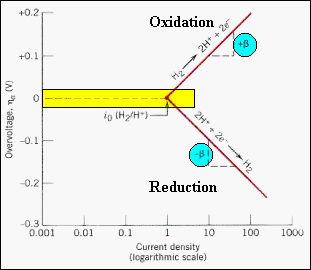
| ·
Using:
Ln {i(η)/(
ie)} = η(ne)/kT
= βη , the
activation energy controlled behavior of a hydrogen electrode for both
oxidation and reduction reactions can be displayed on a single plot.
· At zero overpotential, the exchange current density for the two reactions is the same. No net current flows. As the overpotential is made positive or negative, either the reduction or oxidation reaction will dominate, and the current density will increase logarithmically with the overpotential. · The slope of each branch is β = (ne/kT). The positive branch corresponds to the oxidation reaction, and the negative branch to the reduction reaction. · For an overpotential change of 0.2 volts, the diagram shows that the oxidation or reduction rates changes by two orders of magnitude! |
|||||||||||||||
 |
|||||||||||||||
| From:
Callister,
"Materials Science & Engineering." Wiley (2000) |
|||||||||||||||