|
|
·
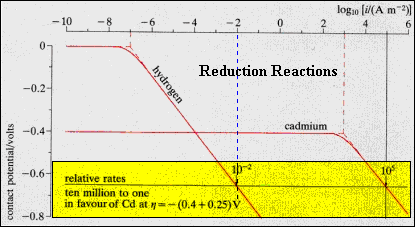
The
equilibrium exchange current for each system is shown by the vertical dotted
line, and is 10 orders of magnitude smaller for hydrogen than cadmium.
Increasing the overpotential above zero volts, the H+ reduction
rate increases linearly, the Cd rate remaining at the equilibrium value
until its electrode potential, -0.4V, is reached. Above this threshold,
the Cd2+ reduction rate increases linearly with voltage.
·
At
the overpotential of -0.65 V, the Cd-rate is 10+5
and the H-rate is 10-2,
a seven order of magnitude difference. H-liberation will, therefore, be
unimportant for this system. |
|