|
·
For
Aluminum deposition from an oxygen-free molar acid aqueous solution, hydrogen
release dominates the overall reaction.
·
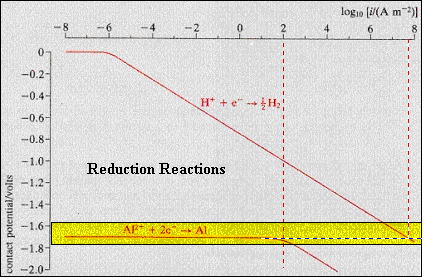
As
shown, aluminum has an electrode potential of - 1.66 V and an exchange
current of 100 A/m2.
The hydrogen production rate at the overpotential required to initiate
aluminum deposition is 106
times larger than the aluminum production rate.
·Electrolytic
plating of aluminum from acidic electrolytes is not possible and alkaline
electrolytes dissolve the aluminum.
·
Aluminium
can be electrolytically recovered from Al2O3
using the solid electrolyte (Na3AlF6
+ CaF2 )
at temperatures of about 1200 K. |
|
|
|
|
|
|
|
|
|
|
|