|
Crevice
Corrosion
· Composition
differences between regions of an electrolyte gives rise to different electrode
potentials with the same material, and hence local corrosion.
·
A
dilute electrolyte region gives rise to a more anodic electrode than a
concentrated electrolyte. The reaction: M
< - > M2+
+ 2e goes
from left to right in a dilute system and from right to left in a concentrated
system, measured in comparison to the equilibrium concentration.
·
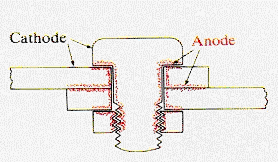
In
the structure illustrated, the transport of electrolyte to the interior
of the crevice is restricted and its local concentration is below that
at the exterior surfaces. Anodic corrosion, therefore, occurs in the crevice
region and is hard to detect by exterior inspection. |
|
|
|
|
|
|
|
|
|
|
|
|