|
·
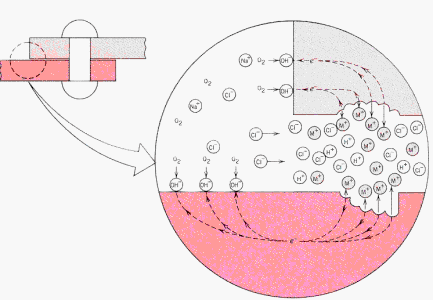
Oxygen
concentration cells are important in crevice corrosion in a moist atmosphere
where the reduction reaction:
2H2O
+ O2 (soln)
+ 4e (cathode) -> 4 (OH)-(soln)
occurs. Anodic
corrosion takes place most rapidly in oxygen deficient areas. The interior
region is, therefore, anodic and the exterior region with the higher oxygen
content is cathodic.
· The
diagram illustrates this behavior for the riveted junction between two
metal plates in an oxygenated NaCl electrolytic environment (sea water).
Metal ions are dissolved in the interior, anodic, region and hydroxyl ions
are formed by electron transfer at the exterior, cathodic, surfaces. HCl
formation in the crevice also accelerates corrosion. |
|