|
CO2 to Fuel – a Mirage or Reality? by Yuan Hu (Chemistry) |
|
| |
|
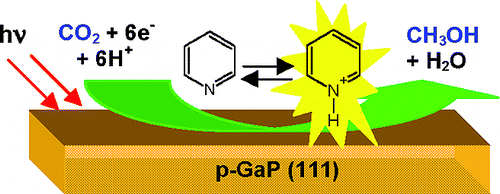
We inhale, then exhale, breathing out a stable chemical – CO2. Atmospheric CO2 is converted back to hydrocarbons by the virtue of photosynthesis carried out by plants. If you realize, the whole cycle is simply a trick to make and break various chemical bonds on carbon atoms. Nevertheless, for hundreds of millions years, solar energy has been renewably harvested and be stored via these chemical reactions. Eventually, this energy is delivered to diverse digestion systems of the creatures on this planet and gets released again to sustain their livings. Throughout the course of the history, a tremendous amount of solar energy has been stored biochemically and reserved deep underground as fossil fuels. However, in the past a few hundred years, human realized the gigantic energy inside the fuels, and then started liberating massive energy by burning them crudely and greedily. Today, we have electricity, we can travel around the world in one day, we believe that we are dominating the world, and in the meantime we are putting us at a falling edge. This subtle carbon balance has been undermined since the beginning of Industrial Revolution – the enormous quantity of CO2 emitted due to fossil fuels combustion and the deforestation have continuously increased the concentration of CO2 in the atmosphere, and there is no sign of slowing down. Many evidences have manifested that the increasing content of CO2 in the atmosphere is intimately related to drastic climate changes in the past decades. On the other hand, we are squandering and depleting fossil fuels so blindly, whilst the energy stored by plants over millions of years may very likely to run out before human society undergoes a safe switch to another reliable energy source. If carbon is recyclable, and if plants are cleaning our waste tirelessly, as the dominant creature of this earth, shouldn’t we act more responsibly for our home? Green is not just herb’s duty. Scientists are pioneers in fulfilling this commitment. Impressive progresses have been made in searching for measures to address the problem. Among all the proposed means, such as carbon sequestration, carbon fixation and so forth, artificial photosynthesis mimics the nature in the closest manner. Apparently, this idea means to convert CO2 to carbon based chemicals of high-energy density with the power of light. Plants do photosynthesis thanks to chloroplast, which is a highly efficient organic device developed through eras of evolution. It’s an extremely tough task to reproduce this process in the lab, especially without the aid of any enzyme and organ to provide an adaptable environment for the successive reactions to remove oxygens from CO2 and insert hydrogens back to carbon. In the lab, the photo energy is harvested using either a molecule-based photosensitizer, or a semiconductor. The number of available molecular energy states for accepting energized electrons in the former is quite limited, leaving nearly no room for proceeding consecutive electron transfers to carbon-based intermediates possessing varied LUMO* values. Hence, a more realistic model for molecule-based photocatalyst capable of reducing CO2 to high-ordered reduced products is a transition metal complex consisting of multiple metal centers holding excited electrons, which is ready to execute a series of electron transfers to a CO2 target. There is no doubt that the chance of finding such a system is slim at present. (*LUMO – lowest unoccupied molecular orbital) Semiconductor materials benefit from the fact that the energy of excited electrons in its conduction band is not a single value, but a spectrum. This means small change in the CO2 energetics along the reaction coordinate is tolerable within the energy distribution of conduction band electrons. In addition, the thermodynamic driving force caused by the difference in ∆G between the Fermi level of the semiconductor and the dominant redox potential of the solution species leads to a band bending. In a p-type semiconductor, the band bending generated at the interface depletes major charge carriers, and create an electrical field driving electrons from the bulk to the solution. All these features favor a series of CO2 reductions occurring in the vicinity of the semiconductor surface. Certainly, the drawbacks in semiconductors are significant as well. For instance, most semiconductors are chemically unstable in aqueous electrolytes, not to mention upon illumination, the photo-induced degradation or corrosion is often devastating to the system. Different from molecule-based photocatalysts which can be uniformly dispersed in the solution, and carry out CO2 reduction homogeneously, CO2 reduction in a semiconductor based system happens exclusively on the surface, and the kinetics is usually limited by the heterogeneous charge transfer. Gaining and losing always come in pair. In an overall process of reducing CO2 to methanol – a chemical fuel can be directly combusted in specifically designed internal combustion engines or a methanol fuel cell – the most difficult step is to transfer the first electron into the LUMO* of the CO2 molecule. In chemists’ language, the molecular orbitals of CO2 is sp hybridized while CO2●- (one electron reduced CO2) is sp2 hybridized. Adding one electron to a neutral CO2 is associated with an extremely unfavorable geometric change of the O-C-O bond angle from 180° to nearly 120°, which requires extensive energy input. Therefore, kinetically the first electron transfer establishes a huge kinetic barrier along the reaction pathway. Although photo-induced separation of electron and hole pairs can gain an inherent potential in the semiconductor, to date no semiconductor have been proven can solely reduce CO2 driven by solar energy without applying a potential bias. Hence the key is to overcome the kinetic barrier, otherwise the journey from CO2 to methanol can be eternally sluggish. Unlike the nature, evolution in artificial synthesis happened in a very drastic manner. In 1994, a big breakthrough in overcoming the kinetic barrier has been achieved in Bocarsly’s lab. What they did was merely adding an organic molecule into the reaction solution, however this extra step totally changed the story.
Figure 1. Benzene and Pyridine. Pyridine has an electron lone pair on its nitrogen.
Pyridine, as shown in Figure 1, is a heterocyclic organic and an electrocatalyst. Even if you are not in chemistry major, do you know benzene? The difference between benzene and pyridine is that a carbon is replaced by nitrogen on the 6-member ring. Do not belittle this nitrogen, since it has an electron lone pair, it can be easily protonated in a slightly acidic solution, where benzene remains intact under the same condition. The protonation is believed to facilitate the acceptance of one electron from a cathode onto the pyridine ring. Then we have this magic one-electron reduced pyridine species (namely pyridinyl radical). According to a calculation study, when a pyridinyl radical meets a free CO2 in aqueous solution, it interacts with CO2 by binding the carbon via its nitrogen to form a carbamate intermediate. Notice that in the meantime the binding bends the linear CO2 to the geometry with an O-C-O bond angle close to 120°, hence the sp orbitals rehybridize to sp2 orbitals. The electron on the pyridine ring then flows on to the bent CO2 moiety, following by another electron transfer and a proton transfer to generate a formate, which is a 2-electron reduced CO2 product. Without pyridine as a catalyst, the bending is achieved on the cathode by raising the potential bias up to as negative as -2.1 V vs. SCE*, whereas with the aid of this small molecular catalyst, the highest potential bias is lowered to the reduction of pyridinium, which is approximately only -0.5 V vs. SCE on platinum surface. Moreover, when pyridine is regenerated after one catalytic cycle, it gets protonated and loaded with an electron again, and acts as a shuttle to carry out a series of electron transfer towards the ending product methanol. Quite amazingly, when this chemistry is applied onto a p-GaP surface (a III-V semiconductor), the reaction can be driven by solar energy at a underpotential of 300 mV, and the faradaic efficiency* approaches unity. *SCE – saturated calomel reference electrode.
Figure 2. A partial pathway of the pyridinium catalyzed CO2 reduction. A carbamate intermediate formed by a one-electron reduced pyridinium and a bent CO2 is shown in the second last step. There is still a large room for improvement in this chemistry, such as adding functional groups onto pyridine to tune its electronic structure, using smaller bandgap semiconductors to utilize wavelengths in the visible region, etc. After all, this discovery opened a new page on human’s footprints to the realization of photosynthesis scientifically, as well as the ultimate goal to industrialize a feasible approach to build a renewable cycle from fuel to CO2 then back to fuel. In an optimistic outlook, we will be able to eventually share some burden from our herbal friends, as a payback to the Mother Nature. Reference
Title image: from reference 2.
|
|
Copyright 2012 by Princeton University China Energy Group. All rights reserved. |
