| Corrosion & Environmental Degradation |
|
||||||||||||||
|
|
|
|||||||||||||
| ·
Very
small overpotentials can have a very large effect on the rate of electro-chemical
reactions.
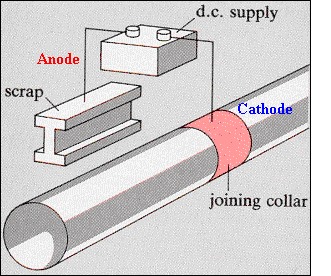
· In using a sacrificial anode material, this potential is provided by the Galvanic cell formed by the two materials, the sacrificial anode and the cathodic material to be protected. · The diagram shows a protection method that involves an applied potential from a suitable DC power supply (battery). One side of the battery is connected to the surrounding medium via the scrap material and the other side to the pipe material to be protected. · Making the pipe negative with respect to the ground provides the desired protection. The current flow is low and only relatively small voltages are required. The corrosion reaction: M -> Mn+ + ne can be stopped or even reversed by the applied potential. |
|||||||||||||||
 |
|||||||||||||||
| From:
Newey and Weaver,
"Materials Principles and Practice," Butterworths (1990) |
|||||||||||||||