| Corrosion & Environmental Degradation |
|
||||||||||||||
|
|
|
|||||||||||||
|
·
A
possible set of reaction paths for the production of free hydrogen gas
at a zinc-electrolyte interface is illustrated. Each of these steps must
occur for the process to be complete.
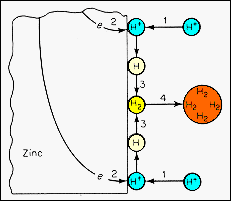
· Each step has an associated activation energy and the slowest of these sequential processes will control the overall rate of cathodic reaction. Overcoming these activation energy barriers is exponentially dependent on the temperature. · In step 1, hydrogen ions from the solution adsorb on the surface and may need to overcome an energy barrier in this process. Desorption will also occur in the steady state. · Step 2 involves electron transfer to the adsorbed ion, producing a neutral hydrogen atom. This atom may diffuse on the surface and react to form hydrogen molecules as shown in step 3. · The hydrogen molecules may also migrate on the zinc surface and enter the electrolyte to form gas bubbles that can leave the system (step 4). |
|||||||||||||||
 |
|||||||||||||||
| From:
M.G. Fontana,
"Corrosion Engineering," Wiley (1986) |
|||||||||||||||