| Corrosion & Environmental Degradation |
|
|||||||||||||||||
|
|
|
||||||||||||||||
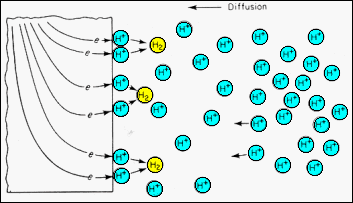
| ·The
supply of hydrogen ions will also depend upon their concentration, i.e.
the pH of the electrolyte. A concentration gradient in the electrolyte
is illustrated.
·If the electrolyte is unstirred, the gradiant causes H-ion diffusion to the surface, where they capture electrons (reduction) to become adsorbed H-atoms. ·The Hydrogen diffusion rate to the surface will be the rate controlling reaction step if the H-ion concentration is low. J = - D0exp{-E/kT}(dc/dx) |
||||||||||||||||||
 |
||||||||||||||||||
| From:
M.G. Fontana, "Corrosion Engineering,"
Wiley (1986) |
||||||||||||||||||
| ·At
higher H-ion concentrations, diffusion
distances may be small and an activated reaction step may control the
overall corrosion process.
· In a flowing or stirred electrolyte, transport to the surface becomes convective. The corrosion rate increases if transport of H-ions to the surface is rate controlling. If the corrosion rate does not change in a stirred system, the overall corrosion reaction rate must depend upon one of the other activation processes discussed above. |
||||||||||||||||||