|
Hydrogen
Cracking
·
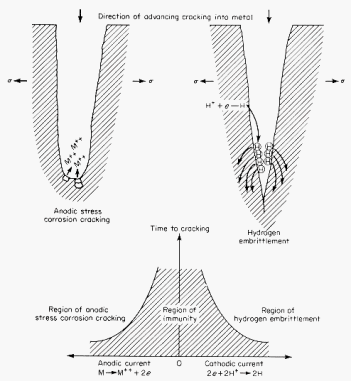
Anodic
or cathodic behavior can lead to different failure processes in the same
material as illustrated in the diagram.
· In
the case where the crack exhibits anodic behavior, stress corrosion cracking
(LHS) dominates crack propagation and material failure. Anodic dissolution
of metal in the region of the crack tip maintains a sharp tip with its
associated high stress concentration.
·
With
cathodic behavior, hydrogen atoms created in the cathodic reaction cause
hydrogen embrittlement,
atomic hydrogen entering the metal, changing crack surface energies, and
inhibiting dislocation motion. The latter makes the crack tip region behave
in a brittle manner, even if the bulk material is ductile. A few parts
per million of hydrogen in the metal can cause cracking. |
|
|
|
|
|
|
|
|
|
|
|
|