|
Corrosion
Pitting
·
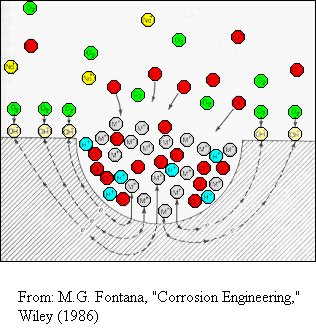
Pit
corrosion is an autocatalytic process in which the corrosion products promote
further corrosion reactions. This is shown for a metal in an oxygenated
NaCl electrolyte. The pit is anodic and the metal surface is cathodic.
· The
localized production of positive metal ions in the pit gives a local excess
of positive charge which attracts the negative chlorine ions from the electrolyte
to produce charge neutrality.
·
The
pit contains a high concentration of MCl molecules which react with water
to produce HCl, the metal hydroxide, and H+
ions, accelerating the corrosion process.
·
In
the pit, the oxygen concentration is essentially zero and all of the cathodic
oxygen reactions take place on the metal surface outside the pit. The pit
is anodic and the locus of rapid dissolution of the metal. |
|
|
|
|
|
|
|
|
|
|