The enzyme Nocturnin, which governs daily tasks such as fat metabolism and energy usage, works in an entirely different way than previously thought, reported a team of researchers at Princeton University.
The newly discovered mechanism reveals the molecular link between the enzyme's daily fluctuations and its energy-regulating role in the body, according to a study published this week in Nature Communications.
"The realization that Nocturnin works in this manner will guide our thinking about sleep, oxidative stress and metabolism, and eventually may serve as a step toward finding better treatments for metabolic diseases," said Alexei Korennykh, an associate professor of molecular biology at Princeton, who led the work.

A team of Princeton researchers discovered how the enzyme Nocturnin (red), best known for its role in fat metabolism and circadian rhythm, acts on NADPH (yellow), to affect energy regulation and metabolic functions. The discovery sheds light on how the enzyme's daily fluctuations help control energy regulation in the body.
Nocturnin is part of the circadian clock that alters the metabolism and behavior of living organisms to match the body's needs at different times of the day. For example, Nocturnin levels fluctuate throughout the day, dramatically peaking when the body first awakens. Nocturnin is also a critical regulator of metabolism; compared to regular mice, mice lacking the enzyme make less insulin, are protected from fatty liver disease and are less susceptible to weight gain.
The precise function of Nocturnin inside cells has remained unclear, however. For many years, the enzyme was thought to turn on and off cellular metabolism by degrading certain cellular messages made of ribonucleic acid, or mRNAs. Last year, however, three groups of researchers — a group from the University of Michigan, a group from the University of Minnesota, and Korennykh's team — discovered that Nocturnin is incapable of degrading RNAs.
To find out how Nocturnin can have such large effects on the body's metabolism, Korennykh teamed up with Princeton's Joshua Rabinowitz, a professor of chemistry and the Lewis-Sigler Institute for Integrative Genomics, and Paul Schedl, a professor of molecular biology. The study was led by postdoctoral research associate Michael Estrella and graduate student Jin Du in the Korennykh lab, and postdoctoral research associate Li Chen in the Rabinowitz lab.
Using methods pioneered by Rabinowitz to screen tissues for the presence of metabolites, the researchers discovered that Nocturnin plays a far more direct role in metabolism than previously appreciated. Rather than degrading mRNAs, the enzyme regulates specific metabolites that help energy production and protect cells from damage. The study determined that Nocturnin is located in the cell's energy-producing structures, the mitochondria, suggesting that this is where the enzyme performs its function.
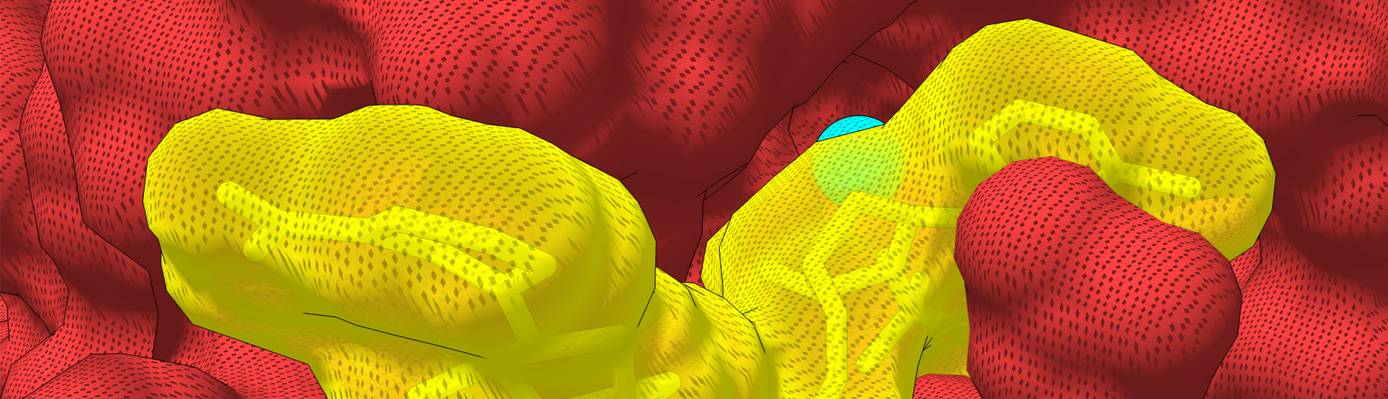
Close-up view of the structure of Nocturnin (red) interacting with NADPH (yellow).
The team found that Nocturnin removes a phosphate group from two molecules important in metabolism, called NADP+ and NADPH. These molecules allow the cell to modulate the levels of reactive oxygen species, which function both as harmful agents causing damage and as signaling molecules controlling metabolism and fat storage. The researchers conclude that Nocturnin is the first known enzyme to perform this reaction on NADP+ and NADPH inside mitochondria.
Removing phosphate groups from NADP+ and NADPH produces two different but equally important molecules, NAD+ and NADH, which are essential for the function of metabolic enzymes — the molecular machines that produce energy by breaking down energy-rich biomolecules such as glucose.
Nocturnin upregulation when an animal first awakens might therefore kick the body's energy production into high gear by providing more NAD+ and NADH. "It is tempting to propose that one physiologic function of Nocturnin could be to maximize available NAD+ and NADH for energy generation in a search for food, using the elevated blood sugar that animals have at the time of awakening," Korennykh said.
Korennykh and colleagues also deciphered the crystal structure of human Nocturnin bound to NADPH, showing at the atomic level how the reaction mediated by Nocturnin occurs. NADPH fits perfectly into Nocturnin's active site so that the enzyme can easily remove the molecule's phosphate group.
Finally, the researchers determined that the fruit fly version of Nocturnin, known as Curled, is also unable to cleave RNA. Instead, Curled uses the same mechanism as human Nocturnin and targets NADP+ and NADPH. The Curled gene was first described over 100 years ago by Thomas Hunt Morgan, the pioneering geneticist who won a Nobel Prize for demonstrating that genes are carried on chromosomes. Though Curled has been studied by fruit fly researchers ever since, its biochemical mechanism was a mystery until now.
"Our work shows that even in the age of genomics and personalized medicine, basic biology still remains to be understood," Korennykh said. "In the example of Nocturnin and Curled, a pathway regulating some of the most important molecules in metabolism was hidden in plain sight for the past 100 years."
The study, "The Metabolites NADP+ and NADPH are the Targets of the Circadian Protein Nocturnin (Curled)," by Michael A Estrella, Jin Du, Li Chen, Sneha Rath, Eliza Prangley, Alisha Chitrakar, Tsutomu Aoki, Paul Schedl, Joshua Rabinowitz and Alexei Korennykh, was published online in Nature Communications on May 30, 2019. DOI: 10.1038/s41467-019-10125-z. This study was funded by Princeton University, the National Institutes of Health (grants 1R01GM110161, 5T32GM007388, F99 CA212468-01, 1DP1DK113643, R01 CA163591, P30 CA072720, R35 GM126975, P41GM111244), the Burroughs Wellcome Foundation (grant 1013579), the Vallee Foundation, the Stand Up to Cancer — Cancer Research UK — Lustgarten Foundation Pancreatic Cancer Dream Team collective (grant SU2C-AACR-DT-20-16), and the U.S. Department of Energy (grants KP1605010, DE-SC0012704).



